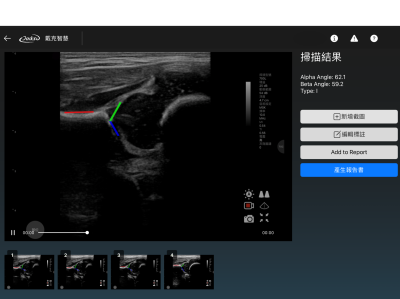
Daikso Cardiac Ultrasound Imaging Marking Aided System
Daikso CO.,LTD.
Smart Hospital Solution Community service care system AI artificial intelligence Big data analysis APP Software Medical computer and tabletInformation
Specification
This product does not include any hardware. Please refer to the following minimum software and hardware requirements (or above):
Operating System: iPadOS 16, iOS 14, API Level 21
Processor: A12Z Bionic Chip, A14, Snapdragon 888
Memory: 6GB RAM
Storage: 64GB internal storage
Benefits
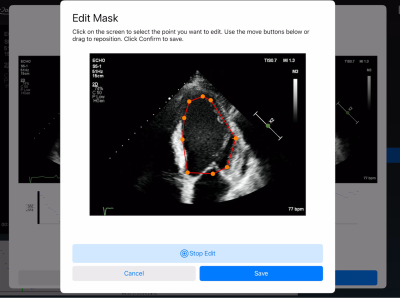
Specifically Designed for Cardiac Ultrasound
Optimized interface and features tailored to the characteristics of cardiac imaging, enhancing annotation efficiency and diagnostic accuracy.
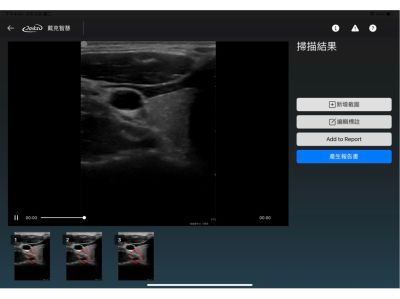
· Support for Multiple Image Formats
Compatible with various medical image formats such as DICOM, JPEG, and PNG, ensuring high interoperability with existing imaging systems.
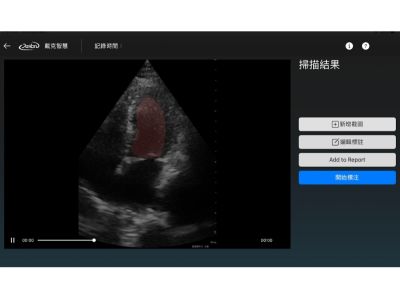
· Visualized Image Display Platform
Offers a clear and interactive interface for image visualization, assisting clinicians in accurate interpretation and manipulation.
· Annotation and Processing Tools
Built-in basic image processing tools (e.g., zoom, resize, contrast adjustment) with annotation support to improve labeling efficiency.
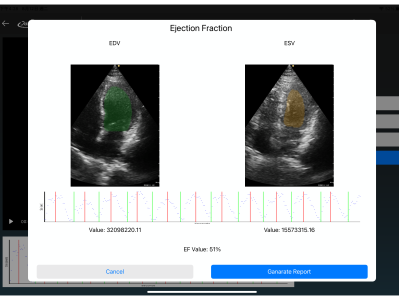
· Automated Export of Annotated Reports
Annotation results can be exported in report formats, facilitating storage, review, and integration with other systems.
Reduces manual operations and documentation time for physicians, accelerating diagnosis and report generation.
Business Partners
GE HEALTHCARE、Aco Healthcare Co., Ltd、TRUST BIO-SONICS INC.
Success Stories
Top 10 in 2025 Computex InnoVEX Selection
Medical Device Manufacturing License – QMS2351 and QMS5518, Ministry of Health and Welfare
"Daikso" Medical Image Label System (Non-Sterile) (Taiwan FDA – MOHW-MD-No.010221)
”Daikso” Cardiac Ultrasound Imaging Marking Aided System (Non-Sterile) (Taiwan FDA – MOHW-MD-No. 010267)
”Daikso” Hip Joint Ultrasound Imaging Marking Aided System(Non-Sterile) (Taiwan FDA – MOHW-MD-No. 010275)
”Daikso” Thyroid Ultrasound Imaging Marking Aided System(Non-Sterile) (Taiwan FDA – MOHW-MD-No. 010293)
Approved in 2023 by the Ministry of Digital Affairs: AI Program II (Total Funding: NTD 12M)
Medical Device Manufacturer License (MD6101007063)
Daikso’s core technologies originate from extensive hands-on experience in medical imaging AI, and have been recognized by multiple world-class competitions, including:
Ranked No. 1 2022 MICCAI DFUC
Finalists in Top Five Finalist - EndoCV 2022
Ranked No. 1- "Paper with Code" in 2021
8th place(1200+ teams) in validation phase - BraTS 2021, MICCAI
Ranked No. 1 Paper with Code AI Ranking, 2021
Ranked No. 1 Adaptive Computing Challenge 2021, AMD-Xilinx
The only 1 polyp detection AI library serving in AMD-Xilinx AI Library
Ranked No. 2- MedAI 2021 in instrument segmentation
Ranked No. 3 - MedAI 2021 in polyp segmentation
National/International Medical Device Legal Permit No.
Taiwan FDA – MOHW-MD-No.010267