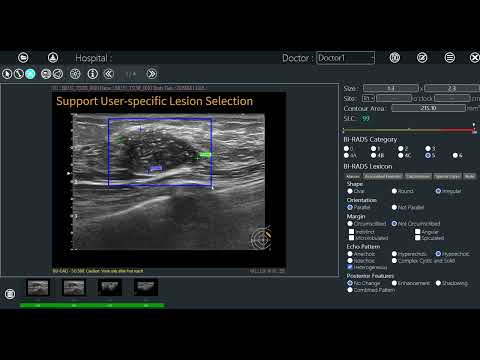
Explore BR-Viewer, your AI assistant, to help interpret 3D Automated Breast Ultrasound (ABUS) images!
1. Fast analysis, accurate diagnosis: With AI technology, BR-Viewer can rapidly analyze a large number of ABUS images, assisting physicians in quickly identifying tumors and abnormal structures. This enables accurate analysis and diagnosis to be completed in a short time, effectively reducing interpretation time.
2. Multi-angle image analysis: BR-Viewer provides image analysis from different perspectives, allowing physicians to view suspicious lesions from multiple angles, aiding in a more comprehensive understanding of the condition.
3. Professional structured reporting: BR-Viewer supports structured reporting, allowing physicians to easily select or deselect images for report generation and annotate and review completed results. This makes generating professional structured reports convenient and quick.
4. Compatible with WorkstationOne: BR-Viewer supports WorkstationOne users as a plugin, providing users with more convenience and options.